2012年1月26日 星期四
2012年1月13日 星期五
Colloids and Surfaces A: Physicochemical and Engineering Aspects, Volume 70, Issue 3, 31 March 1993, Pages 253–268

Volume 70, Issue 3, 31 March 1993, Pages 253–268

- J. Cihlář
- Technical University of Brno, Technická 2, 616 69 Brno, Czechoslovakia
- Received 15 June 1992. Accepted 15 September 1992. Available online 7 November 2001.
Making Silica Aerogels
Making Silica Aerogels
http://eetd.lbl.gov/ecs/aerogels/sa-making.html
The discussion below relies upon the following terms:
- Hydrolysis:
- The reaction of a metal alkoxide (M-OR) with water, forming a metal hydroxide (M-OH).
- Condensation:
- A condensation reaction occurs when two metal hydroxides (M-OH + HO-M) combine to give a metal oxide species (M-O-M). The reaction forms one water molecule.
- Sol:
- A solution of various reactants that are undergoing hydrolysis and condensation reactions. The molecular weight of the oxide species produced continuously increases. As these species grow, they may begin to link together in a three-dimensional network.
- Gel Point:
- The point in time at which the network of linked oxide particles spans the container holding the Sol. At the gel point the Sol becomes an Alcogel.
- Alcogel (wet gel):
- At the gel point, the mixture forms a rigid substance called an alcogel. The alcogel can be removed from its original container and can stand on its own. An alcogel consists of two parts, a solid part and a liquid part. The solid part is formed by the three-dimensional network of linked oxide particles. The liquid part (the original solvent of the Sol) fills the free space surrounding the solid part. The liquid and solid parts of an alcogel occupy the same apparent volume.
- Supercritical fluid:
- A substance that is above its critical pressure and critical temperature. A supercritical fluid possesses some properties in common with liquids (density, thermal conductivity) and some in common with gases (fills its container, does not have surface tension).
- Aerogel:
- What remains when the liquid part of an alcogel is removed without damaging the solid part (most often achieved by supercritical extraction). If made correctly, the aerogel retains the original shape of the alcogel and at least 50% (typically >85%) of the alcogel's volume.
- Xerogel:
- What remains when the liquid part of an alcogel is removed by evaporation, or similar methods. Xerogels may retain their original shape, but often crack. The shrinkage during drying is often extreme (~90%) for xerogels.
Sol-Gel Chemistry
The formation of aerogels, in general, involves two major steps, the formation of a wet gel, and the drying of the wet gel to form an aerogel. Originally, wet gels were made by the aqueous condensation of sodium silicate, or a similar material. While this process worked well, the reaction formed salts within the gel that needed to be removed by many repetitive washings (a long, laborious procedure). With the rapid development of sol-gel chemistry over the last few decades, the vast majority of silica aerogels prepared today utilize silicon alkoxide precursors. The most common of these are tetramethyl orthosilicate (TMOS, Si(OCH3)4), and tetraethyl orthosilicate (TEOS, Si(OCH2CH3)4). However, many other alkoxides, containing various organic functional groups, can be used to impart different properties to the gel. Alkoxide-based sol-gel chemistry avoids the formation of undesirable salt by-products, and allows a much greater degree of control over the final product. The balanced chemical equation for the formation of a silica gel from TEOS is:Si(OCH2CH3)4 (liq.) + 2H2O (liq.) = SiO2 (solid) + 4HOCH2CH3 (liq.)
The above reaction is typically performed in ethanol, with the final density of the aerogel dependent on the concentration of silicon alkoxide monomers in the solution. Note that the stoichiometry of the reaction requires two moles of water per mole of TEOS. In practice, this amount of water leads to incomplete reaction and weak, cloudy aerogels. Most aerogel recipes, therefore, use a higher water ratio than is required by the balanced equation (anywhere from 4-30 equivalents).Catalysts
The kinetics of the above reaction are impracticably slow at room temperature, often requiring several days to reach completion. For this reason, acid or base catalysts are added to the formulation. The amount and type of catalyst used play key roles in the microstructural, physical and optical properties of the final aerogel product.Acid catalysts can be any protic acid, such as HCl. Basic catalysis usually uses ammonia, or, more commonly, ammonia and ammonium fluoride. Aerogels prepared with acid catalysts often show more shrinkage during supercritical drying and may be less transparent than base catalyzed aerogels. The microstructural effects of various catalysts are harder to describe accurately, as the substructure of the primary particles of aerogels can be difficult to image with electron microscopy. All show small (2-5 nm diameter) particles that are generally spherical or egg-shaped. With acid catalysis, however, these particles may appear "less solid" (looking something like a ball of string) than those in base-catalyzed gels.
As condensation reactions progress the sol will set into a rigid gel. At this point, the gel is usually removed from its mold. However, the gel must be kept covered by alcohol to prevent evaporation of the liquid contained in the pores of the gel. Evaporation causes severe damage to the gel and will lead to poor quality aerogels
Single-Step vs. Two-Step Aerogels
Typical acid or base catalyzed TEOS gels are often classified as "single-step" gels, referring to the "one-pot" nature of this reaction. A more recently developed approach uses pre-polymerized TEOS as the silica source. Pre-polymerized TEOS is prepared by heating an ethanol solution of TEOS with a sub-stoichiometric amount of water and an acid catalyst. The solvent is removed by distillation, leaving a viscous fluid containing higher molecular weight silicon alkoxy-oxides. This material is redissolved in ethanol and reacted with additional water under basic conditions until gelation occurs. Gels prepared in this way are known as "two-step" acid-base catalyzed gels. Pre-polymerized TEOS is available commercially in the U.S. from Silbond Corp. (Silbond H-5).These slightly different processing conditions impart subtle, but important changes to the final aerogel product. Single-step base catalyzed aerogels are typically mechanically stronger, but more brittle, than two-step aerogels. While two-step aerogels have a smaller and narrower pore size distribution and are often optically clearer than single-step aerogels.
Aging and Soaking
When a sol reaches the gel point, it is often assumed that the hydrolysis and condensation reactions of the silicon alkoxide reactant are complete. This is far from the case. The gel point simply represents the time when the polymerizing silica species span the container containing the sol. At this point the silica backbone of the gel contains a significant number of unreacted alkoxide groups. In fact, hydrolysis and condensation can continue for several times the time needed for gelation. Failure to realize, and to accommodate this fact is one of the most common mistakes made in preparing silica aerogels. The solution is simple--patience. Sufficient time must be given for the strengthening of the silica network. This can be enhanced by controlling the pH and water content of the covering solution. Common aging procedures for base catalyzed gels typically involve soaking the gel in an alcohol/water mixture of equal proportions to the original sol at a pH of 8-9 (ammonia). The gels are best left undisturbed in this solution for up to 48 hours.This step, and all subsequent processing steps, are diffusion controlled. That is, transport of material into, and out of, the gel is unaffected by convection or mixing (due to the solid silica network). Diffusion, in turn, is affected by the thickness of the gel. In short, the time required for each processing step increases dramatically as the thickness of the gel increases. This limits the practical production of aerogels to 1-2 cm-thick pieces.
After aging the gel, all water still contained within its pores must be removed prior to drying. This is simply accomplished by soaking the gel in pure alcohol several times until all the water is removed. Again, the length of time required for this process is dependent on the thickness of the gel. Any water left in the gel will not be removed by supercritical drying, and will lead to an opaque, white, and very dense aerogel.
Supercritical Drying
 Process conditions for both the carbon dioxide substitution/drying process and the alcohol drying process.
Process conditions for both the carbon dioxide substitution/drying process and the alcohol drying process.At this point the vessel can be opened and the aerogels admired for their intrinsic beauty.
Typical Recipes
Single-Step Base Catalyzed Silica Aerogel
This will produce an aerogel with a density of approx. 0.08 g/cm3. The gel time should be 60-120 minutes, depending on temperature.- Mix two solutions:
- Silica solution containing 50 mL of TEOS, 40 mL of ethanol
- Catalyst solution containing 35 mL of ethanol, 70 mL of water, 0.275 mL of 30% aqueous ammonia, and 1.21 mL of 0.5 M ammonium fluoride.
- Slowly add the catalyst solution to the silica solution with stirring.
- Pour the mixture into an appropriate mold until gelation.
- Process as described above.
Two-Step Acid-Base Catalyzed Silica Aerogel
This will produce an aerogel with a density of approx. 0.08 g/cm3. The gel time should be 30-90 minutes, depending on temperature.- Mix two solutions:
- Silica solution containing 50 mL of precondensed silica (Silbond H-5, or equivalent), 50mL of ethanol
- Catalyst solution containing 35 mL of ethanol, 75 mL of water, and 0.35 mL of 30% aqueous ammonia.
- Slowly add the catalyst solution to the silica solution with stirring.
- Pour the mixture into an appropriate mold until gelation.
- Process as described above.
2012年1月11日 星期三
Synthesis and preliminary characterization of octakis (chloropropyldimethylsiloxy) octasilsesquioxane
Synthesis and preliminary characterization of octakis (chloropropyldimethylsiloxy) octasilsesquioxane
http://www.scielo.br/scielo.php?pid=S1516-14392004000300020&script=sci_arttext
http://www.scielo.br/scielo.php?pid=S1516-14392004000300020&script=sci_arttext
Tetraethyl orthosilicate (TEOS) 矽酸乙酯
| Tetraethyl orthosilicate | |
|---|---|
 | 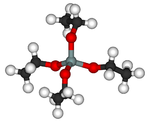 |
性質:Si(OC2H5)4 又名正矽酸乙酯、原矽酸乙酯。無色或淡棕色液體。
略有香味。
密度0.9320。
沸點168.8℃,熔點-82.5℃,折射率1.3928。
在潮濕空氣(水解)中變渾濁。靜置後又澄清而析出矽酸沉澱。
用於製造耐化學品塗料和耐熱塗料。也用作製備有機矽的溶劑。
由四氯化矽和無水乙醇作用後經蒸餾而製得。

Alternatively TEOS can be considered to be the ethyl ester of orthosilicic acid, Si(OH)4. It is a prototypical alkoxide.
製備
TEOS is a tetrahedral molecule. Many analogues exist, and most are prepared by alcoholysis of silicon tetrachloride:
SiCl4 + 4 ROH → Si(OR)4 + 4 HCl
where R = alkyl such as methyl, ethyl, propyl, etc.
水解反應( hydrolysis reaction )
Si(OC2H5)4 + 2 H2O → SiO2 + 4 C2H5OH
This hydrolysis reaction is an example of a sol-gel process. The side product is ethanol.
The reaction proceeds via a series of condensation reactions that convert the TEOS molecule into a mineral-like solid via the formation of Si-O-Si linkages.
Rates of this conversion are sensitive to the presence of acids and bases, both of which serve as catalysts.
The Stöber process allows the formation of monodisperse silica particles.
At elevated temperatures (>600 °C), TEOS converts to silicon dioxide:
Si(OC2H5)4 → SiO2 + 2O(C2H5)2
The volatile coproduct is diethylether.
=======================================================
Stöber process
http://en.wikipedia.org/wiki/St%C3%B6ber_process
太陽能燈具和LED燈具專業英語翻譯
工作環境溫度:Working temperature工作電壓:Supply voltage額定電源頻率Rated power frequency額定功率Rated power驅動電源效率Power supply efficiency功率因數Power-factor(PF)LED發光效率LED luminoue efficiency燈具初始光通量Luminous flux燈具出光效率 Lamp Flux色溫Color temperature顯色指數CRI: Ra>75防護等級IP rating:IP65使用壽命 Working life外殼材質Shell material character產品尺寸 Size(A*B*C mm)重量 Net weight (kg)包裝尺寸 Packing dimensions(mm)
太陽能的相對多一點:
交流電兩種電的形態之一: 交變電流, 常用於住家中.
非晶具有無週期型的原子結構.
非晶矽有時簡稱為'a-矽'作為一種無序半導體材料用於增強等離子體化學蒸汽沉積(PECVD)工藝中. 此工藝可用來在不銹鋼的基片上產生薄膜太陽能發電層.
安培 (Amps)電流單位. 可視為電力流動的數量單位
光伏矩陣或發電板陣(Array - photovoltaic)太陽能發電板串聯或併聯連接在一起形成矩陣.
阻流二極管 (Blocking Diode)用來防止反向電流, 在發電板陣中, 阻流二極管用來防止電流流向一個或數個失效或有遮影的發電板(或一連串的太陽能發電板) 上. 在夜間或低電流出的期間, 防止電流從蓄電池流向光伏發電板矩陣."
光伏發電系統平衡(BOS or Balance of System - photovoltaic)光伏發電系統除發電板矩陣以外的部分. 例如開關, 控制儀表, 電力溫控設備, 矩陣的支撐結構, 儲電組件等等.
旁路二極管 (Bypass Diode)是與光伏發電板並聯的二極管. 用來在光電板被遮影或出故障時提供另外的電流通路.
光伏發電板 (電池) (Cell-photovoltaic)太陽能發電板中最小的組件.
充電顯示器 (表) (Charge Monitor/Meter)用以測量電流安培量的裝置, 安培表.
充電調節器 (Charge Regulator)"用來控制蓄電池充電速度和/或充電狀態的裝置, 連接於光伏發電板矩陣和蓄電池組之間. 它的主要作用是防止蓄電池被光伏發電板過度充電, 同時監控光伏發電矩陣和/或蓄電池的電壓."
組件 (Components)指用於建立太陽能電源系統所需的其他裝置.
交直流轉換器 (Converter)將交流電轉換成直流電的裝置.
晶體狀 (Crystalline)具有三維的重複的原子結構.
直流電 (DC)"兩種電流的形態之一, 常見於使用電池的物件中, 如收音機, 汽車, 手提電腦, 手機等等."
無序結構 (Disordered)減小並消除晶格的局限性. 提供新的自由度, 從而可在多維空間中放置其他元素. 使它們以前所未有的方式互相作用. 這種技術應用多種元素以及復合材料. 它們在位置,移動及成分上的不規則可消除結構的局限性, 因而產生新的局部規則環境. 而這些新的局部環境決定了這些材料的物理性質, 電子性質以及化學性質. 因此使得合成具有新潁機理的新型材料成為可能.
電網連接- 光伏發電(Grid-Connected - photovoltaic)是一種由光伏發電板陣向電網提供電力的光伏發電系統. 這些系統可由供電公司或個別樓宇來運作.
直流交流轉換器 (Inverter)用來將直流電轉換成交流電的裝置.
千瓦 (Kilowatt)1000瓦特, 一個燈泡通常使用40至100瓦特的電力.
百萬瓦特 (Megawatt)1,000,000瓦特
光伏發電板 (Module - photovoltaic)光伏電池以串聯方式連在一起組成發電板.
奧佛電子 (Ovonic)[以SR 奧佛辛斯基(聯合太陽能公司創始人)及電子的組合命名] - 用來描述我們獨有的材料, 產品和技術的術語.
奧佛辛斯基效應 (Ovshinsky effect)一種特別的玻璃狀薄膜在極小電壓的作用下從一種非導體轉變成一種半導體的效應..
並聯連接 (Parallel Connection)一種發電板連接方法. 這種連接法使電壓保持相同, 但電流成倍數增加
峰值輸出功能 (Peak Power)持續一段時間(通常是10到30秒)的最大能量輸出.
光伏 (Photovoltaic - PV)光能到電能的直接轉換.
光伏發電板 (電池) (Photovoltaic Cell)經過特殊處理可將太陽能輻射轉換成電力的半導體材料.
捲到卷工序 (Roll-to-Roll Process)將整捲的基件連續地轉變成整捲的產品的工序.
串聯連接 (Series Connection)電流不變電壓倍增的連接方式.
太陽能 (Solar)來自太陽的能量.
太陽能收集器 (Solar Collectors)用以捕獲來自太陽的光能或熱能的裝置. 太陽收集器用於太陽能熱水器系統中(常見於住家), 而光伏能收集器則是用於太陽能電力系統.
太陽能加熱 (Solar Heating)利用來自太陽的熱能發電的技術或系統. 太陽能收集器用於太陽能熱水器系統中(常見於住家), 而光伏能收集器則是用於太陽能電力系統中
太陽能發電模塊或太陽能發電板(Solar Module or Solar Panel)一些由太陽能發電板單元所組成的太陽能發電板板塊.
穩定能量轉換效率(Stabilized Energy Conversion Efficiency)長期的電力輸出與光能輸入比例.
系統, 平衡系統(Systems; Balance of Systems)"太陽能電力系統包括了光伏發電板矩陣和其它的部件. 這些部件可使這些太陽能發電板得以應用在需要可控直流電或交流電的住家和商業設施中. 用於太陽能電力系統的其它部件包括:接線和短路裝置, 充電調壓器,逆變器, 儀表和接地部件."
薄膜 (Thin-Film)在基片上形成的很薄的材料層.
伏特 (Volts)電動勢能單位. 能促使一安培的電流通過一歐姆的電阻.
電壓 (Voltage)電勢的量.
電壓表 (Voltage Meter)用以測量電壓的裝置.
瓦特 (Watts)用電壓乘以電流的值來衡量的電力度.
英文
ACOne of two types of electricity: Alternating Current; found in homes.
Amorphoushaving an atomic structure that is not periodic.
Amorphous Silicon"Sometimes abbreviated as ""a-Si"", amorphous silicon is used as a disordered semi-conductor material in the plasma-enhanced chemical vapor deposition (PECVD) process used to create thin-film solar cells on a stainless-steel substrate"
Amps"The unit of electrical current. Can be thought of as the ""flow rate"" of electricity."
Array (photovoltaic)modules wired together in series or parallel form an array.
Blocking Diode"Used to prevent undesired current flow. In a PV array, the diode is used to prevent current flow towards a failed or shaded module (or string of modules) or from the battery to the PV array during periods of darkness or low current production. "
BOS or Balance of System (photovoltaic)the parts of a photovoltaic system other than the array. For instance: switches, controls, meters, power conditioning equipment, supporting structure for the array, storage components, etc.
Bypass DiodeA diode connected in parallel with a PV cell to provide an alternative current path in case of cell shading or failure.
Cell (photovoltaic)the smallest unit of a solar module.
Charge Monitor/MeterA device that measures amperage; amp meter.
Charge Regulator" A device that controls the changing rate and/or state of charge for batteries. Wired between a photovoltaic array and a battery bank., its main job is to prevent the battery from being overcharged from the PV array, while monitoring the array and/ or battery voltage."
ComponentsRefers to other devices used and needed when building a solar system
ConverterAn Electroic Device that changes alternating current (ac) to direct current (dc).
Crystallinehaving a repeating atomic structure in all three dimensions.
DC"One of two types of Electricity Direct Current; found in anything that uses batteries. Radios, cars laptops, cell phones, etc."
Disorderedminimizing and lifting of lattice constraints, which provides new degrees of freedom, permitting the placement of elements in multi-dimensional spaces where they interact in ways not previously available. This allows the use of multi-elements and complex materials where positional, translational, and compositional disorder remove restrictions so new local order environments can be generated controlling the physical, electronic, and chemical properties of the material, thereby permitting the synthesis of new materials with new mechanisms.
Grid-Connected (photovoltaic)a photovoltaic (PV) system in which the PV array supplies power to the grid. Systems can be operated by the utility or by individual buildings.
InverterAn Electronic Device that changes direct current (dc) to alternating current (ac).
Kilowatt1000 watts; a light bulb uses 40-100 watts.
Megawatt"1,000,000 watts"
Module (photovoltaic)cells wired together in series form a module.
Ovonic[after SR Ov(shinsky) + (electr)onic] - the term used to describe our proprietary materials, products, and technologies.
Ovshinsky effectThe effect by which a specific glassy thin film switches from a nonconductor to a semiconductor upon application of a minimum voltage.
Parallel Connection"Connection in which the voltage stays the same, but the amperage multiplies."
Peak Powerthe maximum amount of energy available for a sustained period of time, typically 10 to 30 seconds.
Photovoltaic (PV)direct conversion of light into electrical energy.
Photovoltaic CellThe treated semi-conductor material that converts solar irradiance to electricity.
Roll-to-Roll Processa process where a roll of substrate is continuously converted into a roll of product.
Series ConnectionConnection in which the current (amps) stays the same but the voltage multiplies.
SolarEnergy from the sun.
Solar CollectorsA device designed to capture light or heat energy from the sun. Solar thermal collectors are used in solar hot water systems (often found in homes) and photovoltaic collectors are used in solar electric systems
Solar HeatingTechnologies or systems that take advantage of the heat energy coming from the sun. Solar thermal collectors are used in solar hot water systems (often found in homes) and photovoltaic collectors are used in solar electric systems.
Solar Module or Solar PanelA collection of solar cells interconnected to form a solar panel or module.
Stabilized Energy Conversion Efficiencythe long-term ratio of electrical output to light input.
Systems; Balance of Systems"Solar electric systems include the photovoltaic array and the other components that allow these solar panels to be used in homes and commercial facilities where a regulated DC power supply or an AC power supply is required. Components used in solar electric systems include; wire and disconnect devices, charge regulators, inverters, metering, and grounding components."
Thin-Filma very thin layer of material formed on a substrate.
VoltsThe unit of electromotive force that will force a current of one amp through a resistor or one ohm.
VoltageThe measurement of the force of electricity.
Voltage MeterA device that measures voltage.
WattsA measure of electrical power that is determined by multiplying the voltage by the amperage.
太陽能的相對多一點:
交流電兩種電的形態之一: 交變電流, 常用於住家中.
非晶具有無週期型的原子結構.
非晶矽有時簡稱為'a-矽'作為一種無序半導體材料用於增強等離子體化學蒸汽沉積(PECVD)工藝中. 此工藝可用來在不銹鋼的基片上產生薄膜太陽能發電層.
安培 (Amps)電流單位. 可視為電力流動的數量單位
光伏矩陣或發電板陣(Array - photovoltaic)太陽能發電板串聯或併聯連接在一起形成矩陣.
阻流二極管 (Blocking Diode)用來防止反向電流, 在發電板陣中, 阻流二極管用來防止電流流向一個或數個失效或有遮影的發電板(或一連串的太陽能發電板) 上. 在夜間或低電流出的期間, 防止電流從蓄電池流向光伏發電板矩陣."
光伏發電系統平衡(BOS or Balance of System - photovoltaic)光伏發電系統除發電板矩陣以外的部分. 例如開關, 控制儀表, 電力溫控設備, 矩陣的支撐結構, 儲電組件等等.
旁路二極管 (Bypass Diode)是與光伏發電板並聯的二極管. 用來在光電板被遮影或出故障時提供另外的電流通路.
光伏發電板 (電池) (Cell-photovoltaic)太陽能發電板中最小的組件.
充電顯示器 (表) (Charge Monitor/Meter)用以測量電流安培量的裝置, 安培表.
充電調節器 (Charge Regulator)"用來控制蓄電池充電速度和/或充電狀態的裝置, 連接於光伏發電板矩陣和蓄電池組之間. 它的主要作用是防止蓄電池被光伏發電板過度充電, 同時監控光伏發電矩陣和/或蓄電池的電壓."
組件 (Components)指用於建立太陽能電源系統所需的其他裝置.
交直流轉換器 (Converter)將交流電轉換成直流電的裝置.
晶體狀 (Crystalline)具有三維的重複的原子結構.
直流電 (DC)"兩種電流的形態之一, 常見於使用電池的物件中, 如收音機, 汽車, 手提電腦, 手機等等."
無序結構 (Disordered)減小並消除晶格的局限性. 提供新的自由度, 從而可在多維空間中放置其他元素. 使它們以前所未有的方式互相作用. 這種技術應用多種元素以及復合材料. 它們在位置,移動及成分上的不規則可消除結構的局限性, 因而產生新的局部規則環境. 而這些新的局部環境決定了這些材料的物理性質, 電子性質以及化學性質. 因此使得合成具有新潁機理的新型材料成為可能.
電網連接- 光伏發電(Grid-Connected - photovoltaic)是一種由光伏發電板陣向電網提供電力的光伏發電系統. 這些系統可由供電公司或個別樓宇來運作.
直流交流轉換器 (Inverter)用來將直流電轉換成交流電的裝置.
千瓦 (Kilowatt)1000瓦特, 一個燈泡通常使用40至100瓦特的電力.
百萬瓦特 (Megawatt)1,000,000瓦特
光伏發電板 (Module - photovoltaic)光伏電池以串聯方式連在一起組成發電板.
奧佛電子 (Ovonic)[以SR 奧佛辛斯基(聯合太陽能公司創始人)及電子的組合命名] - 用來描述我們獨有的材料, 產品和技術的術語.
奧佛辛斯基效應 (Ovshinsky effect)一種特別的玻璃狀薄膜在極小電壓的作用下從一種非導體轉變成一種半導體的效應..
並聯連接 (Parallel Connection)一種發電板連接方法. 這種連接法使電壓保持相同, 但電流成倍數增加
峰值輸出功能 (Peak Power)持續一段時間(通常是10到30秒)的最大能量輸出.
光伏 (Photovoltaic - PV)光能到電能的直接轉換.
光伏發電板 (電池) (Photovoltaic Cell)經過特殊處理可將太陽能輻射轉換成電力的半導體材料.
捲到卷工序 (Roll-to-Roll Process)將整捲的基件連續地轉變成整捲的產品的工序.
串聯連接 (Series Connection)電流不變電壓倍增的連接方式.
太陽能 (Solar)來自太陽的能量.
太陽能收集器 (Solar Collectors)用以捕獲來自太陽的光能或熱能的裝置. 太陽收集器用於太陽能熱水器系統中(常見於住家), 而光伏能收集器則是用於太陽能電力系統.
太陽能加熱 (Solar Heating)利用來自太陽的熱能發電的技術或系統. 太陽能收集器用於太陽能熱水器系統中(常見於住家), 而光伏能收集器則是用於太陽能電力系統中
太陽能發電模塊或太陽能發電板(Solar Module or Solar Panel)一些由太陽能發電板單元所組成的太陽能發電板板塊.
穩定能量轉換效率(Stabilized Energy Conversion Efficiency)長期的電力輸出與光能輸入比例.
系統, 平衡系統(Systems; Balance of Systems)"太陽能電力系統包括了光伏發電板矩陣和其它的部件. 這些部件可使這些太陽能發電板得以應用在需要可控直流電或交流電的住家和商業設施中. 用於太陽能電力系統的其它部件包括:接線和短路裝置, 充電調壓器,逆變器, 儀表和接地部件."
薄膜 (Thin-Film)在基片上形成的很薄的材料層.
伏特 (Volts)電動勢能單位. 能促使一安培的電流通過一歐姆的電阻.
電壓 (Voltage)電勢的量.
電壓表 (Voltage Meter)用以測量電壓的裝置.
瓦特 (Watts)用電壓乘以電流的值來衡量的電力度.
英文
ACOne of two types of electricity: Alternating Current; found in homes.
Amorphoushaving an atomic structure that is not periodic.
Amorphous Silicon"Sometimes abbreviated as ""a-Si"", amorphous silicon is used as a disordered semi-conductor material in the plasma-enhanced chemical vapor deposition (PECVD) process used to create thin-film solar cells on a stainless-steel substrate"
Amps"The unit of electrical current. Can be thought of as the ""flow rate"" of electricity."
Array (photovoltaic)modules wired together in series or parallel form an array.
Blocking Diode"Used to prevent undesired current flow. In a PV array, the diode is used to prevent current flow towards a failed or shaded module (or string of modules) or from the battery to the PV array during periods of darkness or low current production. "
BOS or Balance of System (photovoltaic)the parts of a photovoltaic system other than the array. For instance: switches, controls, meters, power conditioning equipment, supporting structure for the array, storage components, etc.
Bypass DiodeA diode connected in parallel with a PV cell to provide an alternative current path in case of cell shading or failure.
Cell (photovoltaic)the smallest unit of a solar module.
Charge Monitor/MeterA device that measures amperage; amp meter.
Charge Regulator" A device that controls the changing rate and/or state of charge for batteries. Wired between a photovoltaic array and a battery bank., its main job is to prevent the battery from being overcharged from the PV array, while monitoring the array and/ or battery voltage."
ComponentsRefers to other devices used and needed when building a solar system
ConverterAn Electroic Device that changes alternating current (ac) to direct current (dc).
Crystallinehaving a repeating atomic structure in all three dimensions.
DC"One of two types of Electricity Direct Current; found in anything that uses batteries. Radios, cars laptops, cell phones, etc."
Disorderedminimizing and lifting of lattice constraints, which provides new degrees of freedom, permitting the placement of elements in multi-dimensional spaces where they interact in ways not previously available. This allows the use of multi-elements and complex materials where positional, translational, and compositional disorder remove restrictions so new local order environments can be generated controlling the physical, electronic, and chemical properties of the material, thereby permitting the synthesis of new materials with new mechanisms.
Grid-Connected (photovoltaic)a photovoltaic (PV) system in which the PV array supplies power to the grid. Systems can be operated by the utility or by individual buildings.
InverterAn Electronic Device that changes direct current (dc) to alternating current (ac).
Kilowatt1000 watts; a light bulb uses 40-100 watts.
Megawatt"1,000,000 watts"
Module (photovoltaic)cells wired together in series form a module.
Ovonic[after SR Ov(shinsky) + (electr)onic] - the term used to describe our proprietary materials, products, and technologies.
Ovshinsky effectThe effect by which a specific glassy thin film switches from a nonconductor to a semiconductor upon application of a minimum voltage.
Parallel Connection"Connection in which the voltage stays the same, but the amperage multiplies."
Peak Powerthe maximum amount of energy available for a sustained period of time, typically 10 to 30 seconds.
Photovoltaic (PV)direct conversion of light into electrical energy.
Photovoltaic CellThe treated semi-conductor material that converts solar irradiance to electricity.
Roll-to-Roll Processa process where a roll of substrate is continuously converted into a roll of product.
Series ConnectionConnection in which the current (amps) stays the same but the voltage multiplies.
SolarEnergy from the sun.
Solar CollectorsA device designed to capture light or heat energy from the sun. Solar thermal collectors are used in solar hot water systems (often found in homes) and photovoltaic collectors are used in solar electric systems
Solar HeatingTechnologies or systems that take advantage of the heat energy coming from the sun. Solar thermal collectors are used in solar hot water systems (often found in homes) and photovoltaic collectors are used in solar electric systems.
Solar Module or Solar PanelA collection of solar cells interconnected to form a solar panel or module.
Stabilized Energy Conversion Efficiencythe long-term ratio of electrical output to light input.
Systems; Balance of Systems"Solar electric systems include the photovoltaic array and the other components that allow these solar panels to be used in homes and commercial facilities where a regulated DC power supply or an AC power supply is required. Components used in solar electric systems include; wire and disconnect devices, charge regulators, inverters, metering, and grounding components."
Thin-Filma very thin layer of material formed on a substrate.
VoltsThe unit of electromotive force that will force a current of one amp through a resistor or one ohm.
VoltageThe measurement of the force of electricity.
Voltage MeterA device that measures voltage.
WattsA measure of electrical power that is determined by multiplying the voltage by the amperage.
LED不同應用對LED支架選材的要求
一,LED封裝廠對LED支架的的要求:
LED(可見光)按市場應用可分為:LED顯示屏,LED照明,LED背光源,LED指示燈,汽車照明應用等,不同的應用對LED有不同的要求,而LED支架作為LED最重要的三大原物料之一,對LED的性能有著重要的影響,所以不同LED應用對LED支架的要求也不一樣.
1. LED顯示屏:LED顯示屏可分為LED室外顯示屏和LED室內顯示屏, LED室外顯示屏須具有高亮及較高的防護等級(須具有具有防風、防雨、防水功能),故一般多采用Lamp LED或Display點陣式來實現,面積一般幾十平方米至幾百平方米,成本較低; 而LED室內顯示屏發光點較小,要求色彩一致性好,寬視角,特別是要求混色效果非常好,故一般用SMD LED來實現.因成本較高,所以顯示面積一般幾至幾十平方米.
因室內屏價格較貴,消費者誰都不願意買回去只用過幾千個小時就會因亮度有很大的衰減而變暗或亮度色彩不一.所以室內屏LED封裝時除要求採用較大尺寸且品質好晶片外,還要求LED支架的PPA反射杯長期耐黃變性要好,能在很長時間內保持較高的反射而不會因反射杯變黃而吸光導致亮度急聚衰減,需能抵抗藍光晶片發出的微量紫外光的長期照射而不會在短期內產生老化變黃.所以用於室內屏應用的LED支架大多會選用長期耐黃變性好的PPA材料射出成型,而不是只追求初始的白度和亮度.
2.LED背光源:可分為小尺寸背光源和中大尺寸的背光源.
A小尺寸背光源:一般指應用於手機及MP3,MP4等小尺寸消費性電子產品的LED背光源.因這些小尺寸的背光源一般連續使用時間都不長,而且對長期使用性能均無嚴格要求,對光衰不敏感.所以一般在封裝時大多會選用初始白度(亮度)較好,反射率較高的PPA材料做的LED支架,其對長期耐黃變性要求不像中大尺寸背光源那麼嚴. 所以選用LED支架時會考慮到高初始亮度及低成本雙方面的要求.
B.中大尺寸背光源:一般指LED TV背光,筆記本電腦背光及一些中大尺寸顯示屏背光源. 筆記本電腦背光源及一些中大尺寸顯示屏背光源一般在一天中的使用時間較長,同時成本也較高,無論是誰都不會希望自己買的筆電,顯示屏或LED TV用不到一兩年就出現較大的光衰及亮度色彩不一致,甚至死燈的現象.其對產品的長期使用性能有較嚴格的要求,尤其是LED TV背光.所以在封裝選用LED支架時大多會選用長期耐黃變性好的PPA材料射出成型的支架, 而不應只追求初始的白度和亮度.因長期耐黃變性較好的材料一般都有加光穩定劑,能在較長的時間內抵抗藍光晶片發出的微量紫外光的照射,延緩老化變黃的速度. 而且初始白度好的LED支架和長期耐黃變性好的LED支架在同等條件下封裝出來的產品亮度差異很小(不到5%).(待續.....)
3.LED照明:可分為LED路燈照明,商業照明,景觀照明及室內照明.
A.LED商業照明因其使用時間長(每天點亮12小時以上,甚至有的每天點亮24小時),節能環保,成本回收快而發展迅速,目前多采用大功率及COB LED來實現.亮度高但受限於散熱技術的影響,目前成本較高且使用壽命無法達到預期要求.目前LED封裝選用LED支架時大多會選用初始白度好亮度高的PPA材料支架,因商業照明成本回收快對長期信賴性未有嚴格要求.
B. LED路燈照明及景觀照明均為市政工程照明,每天工作時間一般在12小時以內,在低碳節能,綠色環保及政府的大力支持下得到了快速的發展.出光亮度高,照度好但受散熱技術的影響,目前成本較高且使用壽命無法達到預期要求. 目前大功率LED封裝選用LED支架時大多會選用初始白度好亮度高的PPA材料支架.
C.室內照明(通用照明):目前以T8,T5等為主流,根據不同的功率有不同的尺寸.室內照明使用的LED SMD及High Power都有,目前還是以SMD為主流.室內照明一般會要求出光均勻,一致性好,顯色性好及使用壽命長,但因目前LED品質良莠不齊,以及散熱技術問題和消費觀念問題的影響,目前大多銷往國外市場.但就室內照明的要求,建議LED封裝廠在選用LED支架時使用長期耐黃變性好的PPA材料射出的LED支架,而不要為追求高初始亮度而忽略了其長期信賴性.
因目前國內LED照明市場缺乏行業標準規範,使得LED照明行業進入門坎較低.使得一些封裝企業為了眼前暫時的利益,而不顧產品的品質,使LED使用壽命長的優點變成的LED照明的短板.如果使得普通民眾不再相信LED通用照明,就會導致整個國內LED通用照明市場的崩潰,那將是整個LED行業的滅頂之災.就目前可用來做照明用的LED支架來說,散熱性最好的陶瓷LED支架,長期耐黃變性最好的是LCP塑膠支架.但前者價格昂貴,約為PPA支架的10倍以上,出於成本考量無法大量推廣;後者目前無法做到PPA能達到的白度,初始亮度較差而無法大量推廣.
所以就目前的狀況來說,急需解決的還是LED的出光效率及散熱問題,同時選用長期耐黃變性較好的PPA材料射出成型的LED支架,以保證產品品質.
LED(可見光)按市場應用可分為:LED顯示屏,LED照明,LED背光源,LED指示燈,汽車照明應用等,不同的應用對LED有不同的要求,而LED支架作為LED最重要的三大原物料之一,對LED的性能有著重要的影響,所以不同LED應用對LED支架的要求也不一樣.
1. LED顯示屏:LED顯示屏可分為LED室外顯示屏和LED室內顯示屏, LED室外顯示屏須具有高亮及較高的防護等級(須具有具有防風、防雨、防水功能),故一般多采用Lamp LED或Display點陣式來實現,面積一般幾十平方米至幾百平方米,成本較低; 而LED室內顯示屏發光點較小,要求色彩一致性好,寬視角,特別是要求混色效果非常好,故一般用SMD LED來實現.因成本較高,所以顯示面積一般幾至幾十平方米.
因室內屏價格較貴,消費者誰都不願意買回去只用過幾千個小時就會因亮度有很大的衰減而變暗或亮度色彩不一.所以室內屏LED封裝時除要求採用較大尺寸且品質好晶片外,還要求LED支架的PPA反射杯長期耐黃變性要好,能在很長時間內保持較高的反射而不會因反射杯變黃而吸光導致亮度急聚衰減,需能抵抗藍光晶片發出的微量紫外光的長期照射而不會在短期內產生老化變黃.所以用於室內屏應用的LED支架大多會選用長期耐黃變性好的PPA材料射出成型,而不是只追求初始的白度和亮度.
2.LED背光源:可分為小尺寸背光源和中大尺寸的背光源.
A小尺寸背光源:一般指應用於手機及MP3,MP4等小尺寸消費性電子產品的LED背光源.因這些小尺寸的背光源一般連續使用時間都不長,而且對長期使用性能均無嚴格要求,對光衰不敏感.所以一般在封裝時大多會選用初始白度(亮度)較好,反射率較高的PPA材料做的LED支架,其對長期耐黃變性要求不像中大尺寸背光源那麼嚴. 所以選用LED支架時會考慮到高初始亮度及低成本雙方面的要求.
B.中大尺寸背光源:一般指LED TV背光,筆記本電腦背光及一些中大尺寸顯示屏背光源. 筆記本電腦背光源及一些中大尺寸顯示屏背光源一般在一天中的使用時間較長,同時成本也較高,無論是誰都不會希望自己買的筆電,顯示屏或LED TV用不到一兩年就出現較大的光衰及亮度色彩不一致,甚至死燈的現象.其對產品的長期使用性能有較嚴格的要求,尤其是LED TV背光.所以在封裝選用LED支架時大多會選用長期耐黃變性好的PPA材料射出成型的支架, 而不應只追求初始的白度和亮度.因長期耐黃變性較好的材料一般都有加光穩定劑,能在較長的時間內抵抗藍光晶片發出的微量紫外光的照射,延緩老化變黃的速度. 而且初始白度好的LED支架和長期耐黃變性好的LED支架在同等條件下封裝出來的產品亮度差異很小(不到5%).(待續.....)
3.LED照明:可分為LED路燈照明,商業照明,景觀照明及室內照明.
A.LED商業照明因其使用時間長(每天點亮12小時以上,甚至有的每天點亮24小時),節能環保,成本回收快而發展迅速,目前多采用大功率及COB LED來實現.亮度高但受限於散熱技術的影響,目前成本較高且使用壽命無法達到預期要求.目前LED封裝選用LED支架時大多會選用初始白度好亮度高的PPA材料支架,因商業照明成本回收快對長期信賴性未有嚴格要求.
B. LED路燈照明及景觀照明均為市政工程照明,每天工作時間一般在12小時以內,在低碳節能,綠色環保及政府的大力支持下得到了快速的發展.出光亮度高,照度好但受散熱技術的影響,目前成本較高且使用壽命無法達到預期要求. 目前大功率LED封裝選用LED支架時大多會選用初始白度好亮度高的PPA材料支架.
C.室內照明(通用照明):目前以T8,T5等為主流,根據不同的功率有不同的尺寸.室內照明使用的LED SMD及High Power都有,目前還是以SMD為主流.室內照明一般會要求出光均勻,一致性好,顯色性好及使用壽命長,但因目前LED品質良莠不齊,以及散熱技術問題和消費觀念問題的影響,目前大多銷往國外市場.但就室內照明的要求,建議LED封裝廠在選用LED支架時使用長期耐黃變性好的PPA材料射出的LED支架,而不要為追求高初始亮度而忽略了其長期信賴性.
因目前國內LED照明市場缺乏行業標準規範,使得LED照明行業進入門坎較低.使得一些封裝企業為了眼前暫時的利益,而不顧產品的品質,使LED使用壽命長的優點變成的LED照明的短板.如果使得普通民眾不再相信LED通用照明,就會導致整個國內LED通用照明市場的崩潰,那將是整個LED行業的滅頂之災.就目前可用來做照明用的LED支架來說,散熱性最好的陶瓷LED支架,長期耐黃變性最好的是LCP塑膠支架.但前者價格昂貴,約為PPA支架的10倍以上,出於成本考量無法大量推廣;後者目前無法做到PPA能達到的白度,初始亮度較差而無法大量推廣.
所以就目前的狀況來說,急需解決的還是LED的出光效率及散熱問題,同時選用長期耐黃變性較好的PPA材料射出成型的LED支架,以保證產品品質.
LED射出導線架
LED射出導線架主要材料為PA9T、PPA、LCP,目前主要廠商為DuPont、Kuraray.
LED支架原料;PPA;PA9T;PA66;PA6;LCP;PPS;PA6T;PA46;
PA9T的化學名稱聚對苯二甲酰壬二胺,是尼龍中吸水率最低的品種,僅為0.17%。耐熱溫度可達290度。 PA9T原料物性描述:尼龍系列樹脂中,吸水性最低,尺寸安定性不會因吸水造成尺寸變化及機械強度下降,高耐熱性,280度過錫測試不會產生氣泡,也適用較高使用溫度之無鉛焊錫,流動性佳,適用在薄肉成形,低瓦斯氣,比其它尼龍樹脂少較不容易污染及腐蝕模具,延長模具使用,結晶速度快,冷卻時間短,在高溫環境中,機械強度,剛性下降較少,接合線強度,回收性佳。 PA9T的玻璃化溫度較高(125℃)和高結晶性使其在高溫下仍保質良好的韌性,優於PA66和PA46,耐摩性和摩擦係數小都大大優於其它尼龍,甚至超過POM和LCP。 PA9T另一個極佳的性能是耐化學品和油、醇、酸和二氯化鈣、熱水和其它流體,幾乎超過所有PA,僅比PPS略差,而對燃油的阻隔性是PA6和PA12的十倍,接近ETFE(乙烯-四氯乙烯共聚物)水平,這些優良性能使PA9T十分適用於汽車機罩製品。 PA9T有不含玻纖及含玻纖(33%, 45%, 50%)和防火等規格,對於汽車機罩、電氣電子產品等市場應用極具發展潛力。
聚鄰苯二酰胺(簡稱PPA)樹脂是以對苯二甲酸或鄰苯二甲酸為原料的半芳香族聚酰胺。既有半結晶態的,也有非結晶態的,其玻璃化溫度在255°F左右。非結晶態的PPA主要用於要求阻隔性能的場合;半結晶態的PPA樹脂主要用於注塑加工,也用於其它熔融加工工藝下文主要介紹後者--半結晶態PPA樹脂,特別註明的除外。半結晶態PPAS的熔點約590°F,以不透明矩形切片的形式供應。
由於PPA樹脂的傑出的物理、熱和電性能,尤其是適中的成本,使它有廣闊的應用範圍。這些性能和優良的耐化學性一起,使PPA成為汽車工業許多用途的候選者。趨向更好的空氣動力學車身設計連同更高性能的馬達,將提高發動機箱的溫度,使傳統的熱塑塑料顯得不盡適用。這些新的要求使PPA成為製作下述部件的候選材料之一:汽車前燈反光器、軸承座、皮帶輪、傳感器殼體、燃料管線元件和電氣元件。
電氣元件的發展方向是小型化和高溫團結,如紅外固結和汽相團結,這需要PPA的優越性能。阻燃級PPA具有優良的電性能、很高的HDT值、高的高溫彎曲模量、能以最小的溢料加工成長的薄壁部件,因此適合於製作開關設備。連接件、電刷座和馬達托架。
LED支架原料;PPA;PA9T;PA66;PA6;LCP;PPS;PA6T;PA46;
PA9T的化學名稱聚對苯二甲酰壬二胺,是尼龍中吸水率最低的品種,僅為0.17%。耐熱溫度可達290度。 PA9T原料物性描述:尼龍系列樹脂中,吸水性最低,尺寸安定性不會因吸水造成尺寸變化及機械強度下降,高耐熱性,280度過錫測試不會產生氣泡,也適用較高使用溫度之無鉛焊錫,流動性佳,適用在薄肉成形,低瓦斯氣,比其它尼龍樹脂少較不容易污染及腐蝕模具,延長模具使用,結晶速度快,冷卻時間短,在高溫環境中,機械強度,剛性下降較少,接合線強度,回收性佳。 PA9T的玻璃化溫度較高(125℃)和高結晶性使其在高溫下仍保質良好的韌性,優於PA66和PA46,耐摩性和摩擦係數小都大大優於其它尼龍,甚至超過POM和LCP。 PA9T另一個極佳的性能是耐化學品和油、醇、酸和二氯化鈣、熱水和其它流體,幾乎超過所有PA,僅比PPS略差,而對燃油的阻隔性是PA6和PA12的十倍,接近ETFE(乙烯-四氯乙烯共聚物)水平,這些優良性能使PA9T十分適用於汽車機罩製品。 PA9T有不含玻纖及含玻纖(33%, 45%, 50%)和防火等規格,對於汽車機罩、電氣電子產品等市場應用極具發展潛力。
聚鄰苯二酰胺(簡稱PPA)樹脂是以對苯二甲酸或鄰苯二甲酸為原料的半芳香族聚酰胺。既有半結晶態的,也有非結晶態的,其玻璃化溫度在255°F左右。非結晶態的PPA主要用於要求阻隔性能的場合;半結晶態的PPA樹脂主要用於注塑加工,也用於其它熔融加工工藝下文主要介紹後者--半結晶態PPA樹脂,特別註明的除外。半結晶態PPAS的熔點約590°F,以不透明矩形切片的形式供應。
由於PPA樹脂的傑出的物理、熱和電性能,尤其是適中的成本,使它有廣闊的應用範圍。這些性能和優良的耐化學性一起,使PPA成為汽車工業許多用途的候選者。趨向更好的空氣動力學車身設計連同更高性能的馬達,將提高發動機箱的溫度,使傳統的熱塑塑料顯得不盡適用。這些新的要求使PPA成為製作下述部件的候選材料之一:汽車前燈反光器、軸承座、皮帶輪、傳感器殼體、燃料管線元件和電氣元件。
電氣元件的發展方向是小型化和高溫團結,如紅外固結和汽相團結,這需要PPA的優越性能。阻燃級PPA具有優良的電性能、很高的HDT值、高的高溫彎曲模量、能以最小的溢料加工成長的薄壁部件,因此適合於製作開關設備。連接件、電刷座和馬達托架。
PPStream(PPS) 歐美劇場再度呈現!!!
(保證看到歐美劇場 or 歐美劇集唷!!)
愛看美劇的朋友又有福啦!! 另一個有在更新的美劇的視訊軟體PPTV (已更新部分網友看不到的問題的解決方案)
更新時間: 2011.10.10
最新版本 v2.7.0.1282
最新版本 v2.7.0.1282
歡迎喜好PPS的網友們來更新唷!!
此次提供兩個版本, 歡迎大家選取喜歡的使用唷!!
(這次版本使用方式稍許不同, 請看我以下的教學!! )
(會提供這個是因為告訴大家 pps 封鎖 歐美劇場 後, 要如何觀賞 pps歐美劇場)
2012年1月10日 星期二
驅動下載中心
AMD -驅動下載中心
http://sites.amd.com/us/game/downloads/Pages/downloads.aspx
Intel -驅動下載中心
http://downloadcenter.intel.com/Default.aspx?lang=zht
NVIDIA -驅動下載中心
http://www.nvidia.com.tw/Download/index.aspx?lang=tw
Realtek -驅動下載中心
http://www.realtek.com.tw/downloads/
驅動之家 -驅動下載頻道 http://drivers.mydrivers.com/
http://sites.amd.com/us/game/downloads/Pages/downloads.aspx
Intel -驅動下載中心
http://downloadcenter.intel.com/Default.aspx?lang=zht
NVIDIA -驅動下載中心
http://www.nvidia.com.tw/Download/index.aspx?lang=tw
Realtek -驅動下載中心
http://www.realtek.com.tw/downloads/
驅動之家 -驅動下載頻道 http://drivers.mydrivers.com/
2012年1月7日 星期六
http://search.babylon.com/?AF=100481&babsrc=HP_ss&mntrId=18fd582100000000000000261850c068
http://search.babylon.com/?AF=100481&babsrc=HP_ss&mntrId=18fd582100000000000000261850c068
Universal Extractor 可解壓縮.exe、.msi跟.dbx檔的「萬能解壓縮器」! http://briian.com/?p=5422
Universal Extractor 可解壓縮.exe、.msi跟.dbx檔的「萬能解壓縮器」! http://briian.com/?p=5422
2012年1月6日 星期五
預聚物、調聚物、齊聚物、縮聚物、共聚物、均聚物的概念
預聚物、調聚物、齊聚物、縮聚物、共聚物、均聚物的概念
1、預聚物
聚合度介於單體與最終聚合物之間的一種分子量較低的聚合物,通常指製備最終聚合物前一階段的聚合物。
2、調聚物在聚合反應中,如ktr(鏈轉移速率常數) >>kp(再引發速率常數),則形成聚合度很小的低聚物,這類反應稱做調聚反應,因此這種調聚反應得到的聚合物也稱為調聚物。其分子量較低,一般只有二到十個鏈節,分子的兩端是與調聚劑分子分裂部分結合的。
如果新自由基活性減弱,則再引發相應減慢,會出現緩聚現象,聚合速率和聚合度都將顯著降低。極端的情況是新自由基穩定,難以繼續再引發增長,就成為阻聚作用。
3、齊聚物
又稱低聚物。高分子與低分子的區別在於前者分子量很高,通常將分子量高於約1萬的稱為高分子(polymer),分子量低於約1000的稱為低分子。分子量介於高分子和低分子之間的稱為低聚物(oligomer,又稱齊聚物)。一般高聚物的分子量為104~106,分子量大於這個範圍的又稱為超高分子量聚合物。但是在行業中,比如PAM,分子量在1500~1800萬以上的才稱為超高分子量PAM。
4、縮聚物
生成聚合物時有水或其他簡單分子放出的聚合稱為縮聚,用這種方法合成的聚合物稱為縮聚物。
5、共聚物
兩種或兩種以上的單體或單體與聚合物間進行的聚合稱為共聚,共聚得到的產物即為共聚物。分嵌段共聚物、接枝共聚物、無規共聚物、有規共聚物等。
6、均聚物
由一種單體聚合而成的聚合物稱為均聚物。
PS:英文的“高分子”主要有兩個詞,即polymer和macromolecule。前者又可譯作聚合物或高聚物;後者又可譯作大分子。這兩個詞雖然常混用,但仍有一定區別,前者通常是指有一定重複單元的合成產物,一般不包括天然高分子,而後者指分子量很大的一類化合物,包括天然和合成高分子,也包括無一定重複單元的複雜大分子。
1、預聚物
聚合度介於單體與最終聚合物之間的一種分子量較低的聚合物,通常指製備最終聚合物前一階段的聚合物。
2、調聚物在聚合反應中,如ktr(鏈轉移速率常數) >>kp(再引發速率常數),則形成聚合度很小的低聚物,這類反應稱做調聚反應,因此這種調聚反應得到的聚合物也稱為調聚物。其分子量較低,一般只有二到十個鏈節,分子的兩端是與調聚劑分子分裂部分結合的。
如果新自由基活性減弱,則再引發相應減慢,會出現緩聚現象,聚合速率和聚合度都將顯著降低。極端的情況是新自由基穩定,難以繼續再引發增長,就成為阻聚作用。
3、齊聚物
又稱低聚物。高分子與低分子的區別在於前者分子量很高,通常將分子量高於約1萬的稱為高分子(polymer),分子量低於約1000的稱為低分子。分子量介於高分子和低分子之間的稱為低聚物(oligomer,又稱齊聚物)。一般高聚物的分子量為104~106,分子量大於這個範圍的又稱為超高分子量聚合物。但是在行業中,比如PAM,分子量在1500~1800萬以上的才稱為超高分子量PAM。
4、縮聚物
生成聚合物時有水或其他簡單分子放出的聚合稱為縮聚,用這種方法合成的聚合物稱為縮聚物。
5、共聚物
兩種或兩種以上的單體或單體與聚合物間進行的聚合稱為共聚,共聚得到的產物即為共聚物。分嵌段共聚物、接枝共聚物、無規共聚物、有規共聚物等。
6、均聚物
由一種單體聚合而成的聚合物稱為均聚物。
PS:英文的“高分子”主要有兩個詞,即polymer和macromolecule。前者又可譯作聚合物或高聚物;後者又可譯作大分子。這兩個詞雖然常混用,但仍有一定區別,前者通常是指有一定重複單元的合成產物,一般不包括天然高分子,而後者指分子量很大的一類化合物,包括天然和合成高分子,也包括無一定重複單元的複雜大分子。
凝膠
一種特殊的分散體系﹐其中膠體顆粒或高聚物分子相互連接﹐搭成架子﹐形成空間網狀結構﹐液體或氣體充滿在結構空隙中。其性質介於固體和液體之間﹐從外表看﹐它成固體狀或半固體狀﹐有彈性﹔但又和真正的固體不完全一樣﹐其內部結構的強度往往有限﹐易於破壞。
 分類 凝膠是個總的名稱﹐根據分散相質點的性質(剛性還是柔性)和形成結構時質點間連接的性質(結構的強度)﹐可分為剛性凝膠與彈性凝膠兩大類。多數的無機凝膠﹐如二氧化硅﹑三氧化二鐵﹑二氧化鈦﹑五氧化二釩等屬於前者﹔而柔性的線型高聚物分子形成的凝膠﹐如橡膠﹑明膠﹑瓊脂等屬於後者。也可將凝膠分為可逆凝膠與不可逆凝膠兩大 類。
分類 凝膠是個總的名稱﹐根據分散相質點的性質(剛性還是柔性)和形成結構時質點間連接的性質(結構的強度)﹐可分為剛性凝膠與彈性凝膠兩大類。多數的無機凝膠﹐如二氧化硅﹑三氧化二鐵﹑二氧化鈦﹑五氧化二釩等屬於前者﹔而柔性的線型高聚物分子形成的凝膠﹐如橡膠﹑明膠﹑瓊脂等屬於後者。也可將凝膠分為可逆凝膠與不可逆凝膠兩大 類。
 製備 溶液或固體(乾凝膠)都能形成凝膠。從固體製備凝膠比較簡單﹐乾膠吸收液體膨脹即成﹐通常為彈性凝膠。從溶液製備凝膠須滿足兩個基本條件﹕降低溶解度﹐使固體物質從溶液中成「膠體分散態」析出﹔析出的固體質點既不沉降﹐也不能自由移動﹐而是搭成骨架形成連續的網狀結構。具體的製備方法可以有﹕冷卻膠體溶液﹐產生過飽和溶 液。如 0.5% 瓊脂溶液冷到 35℃ 就形成固體狀膠凍﹔加入非溶劑﹐例如果膠水溶液加入酒精後就形成凝膠﹔加入鹽類﹐適量的電解質加入到膠粒的親水性較強尤其是形狀不對稱的疏液溶膠中﹐即可形成凝膠﹐如五氧化二釩﹑氫氧化鐵等﹔化學反應﹐利用化學反應產生不溶物﹐並控制反應條件可得凝膠﹐如硅膠的製備。
製備 溶液或固體(乾凝膠)都能形成凝膠。從固體製備凝膠比較簡單﹐乾膠吸收液體膨脹即成﹐通常為彈性凝膠。從溶液製備凝膠須滿足兩個基本條件﹕降低溶解度﹐使固體物質從溶液中成「膠體分散態」析出﹔析出的固體質點既不沉降﹐也不能自由移動﹐而是搭成骨架形成連續的網狀結構。具體的製備方法可以有﹕冷卻膠體溶液﹐產生過飽和溶 液。如 0.5% 瓊脂溶液冷到 35℃ 就形成固體狀膠凍﹔加入非溶劑﹐例如果膠水溶液加入酒精後就形成凝膠﹔加入鹽類﹐適量的電解質加入到膠粒的親水性較強尤其是形狀不對稱的疏液溶膠中﹐即可形成凝膠﹐如五氧化二釩﹑氫氧化鐵等﹔化學反應﹐利用化學反應產生不溶物﹐並控制反應條件可得凝膠﹐如硅膠的製備。
據膨脹機理的研究﹐可以認為膨脹過程分為兩個階段﹐第一階段是溶劑分子鑽入凝膠中與大分子相互作用形成溶劑化層﹐此過程時間很短﹐速度快﹔第二階段是液體的滲透作用﹐此過程中凝膠吸收大量液體﹐體積大大增加。在膨脹過程中由於溶劑分子進入凝膠結構中的速度遠大於大分子擴散到液體中的速度﹐使凝膠內外溶液濃度有很大差值 ﹐即溶劑的活度有很大差異﹐產生膨脹壓。此值很可觀﹐例如明膠濃度為50%時﹐膨脹壓為13千克力/厘米﹐66%時為 45千克力/厘米﹐古代埃及人曾利用木頭吸水時產生很大的膨脹壓來開採建造金字塔的石料﹐即所謂濕木裂石。
脫水收縮現象的實際例子很多﹐如人體衰老時皮膚的變皺﹑面製食品的變硬﹑澱粉漿糊的「乾落」等。
溶膠與凝膠
粒徑在1~100 nm 間的膠農顆粒均勻分散在液體中
一網狀交聯結構物,平均分子鏈長度超過數 mrn,孔洞大小平均在數微米左右
- 原料純度高
- 孔徑大小均勻
- 孔隙分布狹窄
- 燒結溫度低
- 原料成本高
- 製備過程之收縮率較大
- 有微孔隙和碳之殘餘
- 薄膜與塗膜 (thin films and coatings)
- 塊狀物 (monoliths)
- 粉粒、晶粒和球形體 (powders, grains, and spheres)
- 纖維 (fibers)
- 複合物 (composites)
- 多孔凝膠和分離膜 (porous gels and separation membranes)
溶膠凝膠法主要包含五個程序:
- 水解與聚縮合反應
- 凝膠化 (gelation)
- 老化 (aging)
- 乾燥 (drying)
- 熱處理 (heat treating)
- DCS (destabilization of colloidal solution ) → DCS 法是以無機鹽或水合金屬氧化物經水解、解膠 (peptized),再經膠體溶液去穩定化 (destabilized),如趕去溶劑以形成凝膠。
- PMU (Polymerization of molecular units) → PMU 法是以醇氧化物經水解,聚合而形成顆粒狀水合氧化物,其顆粒大小約在數百埃或更小。
Partlaw 與 Yoldas 利用系統的密閉性來判定溶膠種類
- 聚合型溶膠 (polymeric Sol): 在密閉系統中會逐漸變成凝膠
- 膠體型溶膠 (colloidal sol or particulate Sol): 需在開放系統中,藉由溶劑發揮而使膠體聚集成凝膠。
反應部分包括水解及聚縮合兩大步驟
M(OR)n + xH2O → M(OH)x(OR)n-x + xROH
水解步驟以酸催化 (acid catalyzed)
- 所得之聚合體小
- 交鏈程度亦低
- 易呈直鏈狀結構
- 所得聚合體較大
- 易呈網狀結構
- 製得之薄膜孔徑也較大
控制水解與聚縮合反應之速率可控制溶膠顆粒之大小及微結構
- 起始物
- 溶劑
- 觸媒
- pH 值
- 溫度
- 化學添加劑
優點
- 改善一般無機材料因孔洞所造成的機械強度不足
- 藉由有機物填充於無機孔洞中,加強材料之機械強度
- 最常使用溶膠凝膠法 (sol-gel method) 合成
- 比傳統之熔融法製造陶瓷複合材料具有較低之溫度: TEOS 在室溫下就可水解,聚縮合而成無機材料,比傳統之熔融法需 1000℃ 以上之高溫燒結相比,溫度明顯降低很多。
- 無所謂界面分離的問題且為均勻透明之材料: 在整個反應系統中,不管是TEOS 之水解過程,或是單體之聚合反應,均在液相中進行較易達到良好之均勻度,且其所形成之高分子與無機相間是以共價鏈的形式存在。
- 改良材料之機械性質: 因為其兼具有機與無機材料的優點,且其物理性質可藉由改變其有機與無機成份的組成來設計。
訂閱:
意見 (Atom)