| Tetraethyl orthosilicate | |
|---|---|
 | 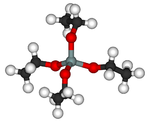 |
性質:Si(OC2H5)4 又名正矽酸乙酯、原矽酸乙酯。無色或淡棕色液體。
略有香味。
密度0.9320。
沸點168.8℃,熔點-82.5℃,折射率1.3928。
在潮濕空氣(水解)中變渾濁。靜置後又澄清而析出矽酸沉澱。
用於製造耐化學品塗料和耐熱塗料。也用作製備有機矽的溶劑。
由四氯化矽和無水乙醇作用後經蒸餾而製得。

Alternatively TEOS can be considered to be the ethyl ester of orthosilicic acid, Si(OH)4. It is a prototypical alkoxide.
製備
TEOS is a tetrahedral molecule. Many analogues exist, and most are prepared by alcoholysis of silicon tetrachloride:
SiCl4 + 4 ROH → Si(OR)4 + 4 HCl
where R = alkyl such as methyl, ethyl, propyl, etc.
水解反應( hydrolysis reaction )
Si(OC2H5)4 + 2 H2O → SiO2 + 4 C2H5OH
This hydrolysis reaction is an example of a sol-gel process. The side product is ethanol.
The reaction proceeds via a series of condensation reactions that convert the TEOS molecule into a mineral-like solid via the formation of Si-O-Si linkages.
Rates of this conversion are sensitive to the presence of acids and bases, both of which serve as catalysts.
The Stöber process allows the formation of monodisperse silica particles.
At elevated temperatures (>600 °C), TEOS converts to silicon dioxide:
Si(OC2H5)4 → SiO2 + 2O(C2H5)2
The volatile coproduct is diethylether.
=======================================================
Stöber process
http://en.wikipedia.org/wiki/St%C3%B6ber_process
沒有留言:
張貼留言